Magnetic suspension balance. Gravimetric sorption in real-world conditions. Dual-sample testing, up to 400°C.

● Fully automated chemisorption analyzer with integrated FTIR detection.
● Enables real-time monitoring of dynamic surface adsorbates during adsorption and desorption.
● Reveals adsorption–reaction synergy and captures transient intermediates for mechanistic insight.
Chemisorption and thermal desorption methods, such as Temperature Programmed Desorption (TPD), are widely utilized for catalyst characterization. These techniques analyze the gases released from a catalyst surface, typically detected using a Thermal Conductivity Detector (TCD) or, in some cases, a mass spectrometer. While they provide valuable insights into the number and strength of active sites, they do not reveal details about the nature of these sites, the type of adsorption, or the presence of multiple adsorption site types.
To address this limitation, the AMI-300 IR integrates standard AMI techniques with real-time catalyst surface analysis using Fourier Transform Infrared (FTIR) spectroscopy. This innovative approach enables direct observation of adsorbed species, offering a deeper understanding of the adsorption and desorption processes.
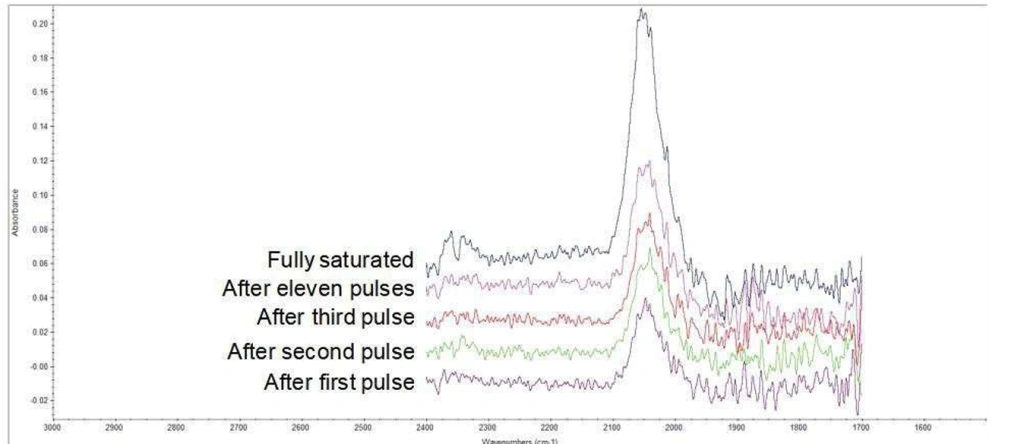
IR detection can also be used during pulse chemisorption procedures to ascertain the mode(s) of adsorption at different coverages. Figure 8 illustrates the adsorption of CO on platinum as the coverage increases. Even at low coverages, all the CO is adsorbed in a single mode, linearly, and there is no evidence for “bridged” CO. These insights are uniquely obtainable through IR spectroscopy, as it directly analyzes the catalyst surface rather than solely monitoring evolved gases.
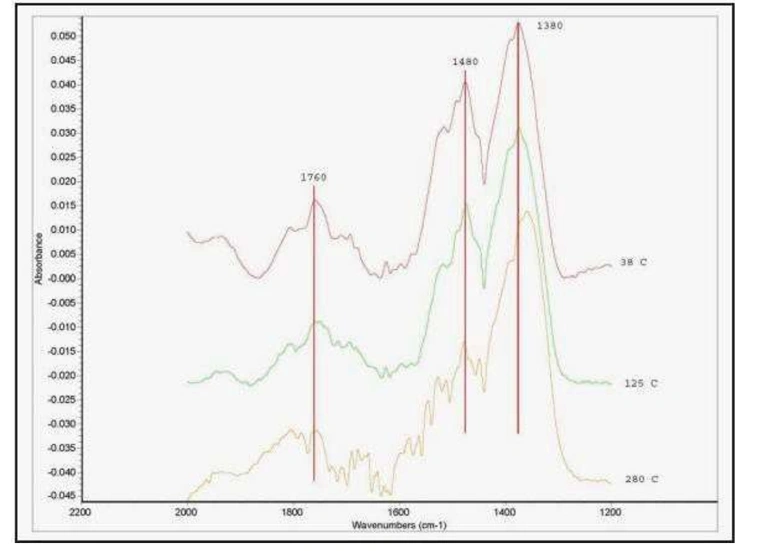
Ammonia can be used as a probe molecule to determine the magnitude and type of acid sites in a catalyst. Below, in figure 9, is an example of ammonia adsorbed on a silica-alumina material. Three broad bands were identified as belonging to the adsorbed ammonia, at approximately 1760, 1480, and 1380 cm-1. The band at 1480 cm-1 can be ascribed to ammonia adsorbed on Brønsted acid sites, the others to ammonia adsorbed on Lewis sites (see for example, M. Niwa et al., J. Phys. Chem. B, 110 (2006) p. 264). By carrying out temperature programmed experiments and following the absorbance of the three bands as a function of temperature, it is possible to measure the isobars for each type of adsorption and assess the strength of each adsorption process. These isobars are shown in figure 10.
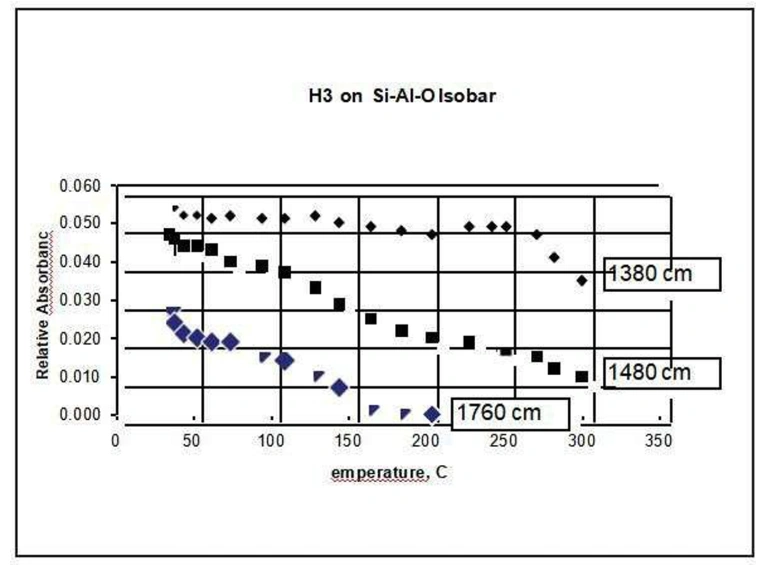
It can be seen from the data above that the adsorption reflected in the 1380 cm-1 band is more strongly held than the other two, perhaps indicating a stronger Lewis-type bond.
The AMI-300 IR expands upon AMI’s line of catalyst characterization instruments, which have been continuously developed and manufactured since 1984. By integrating real-time Fourier Transform Infrared (FTIR) spectroscopy with AMI’s standard detection methods, this system enables researchers to not only quantify the number and strength of active sites but also gain direct insights into the nature of adsorption processes.
