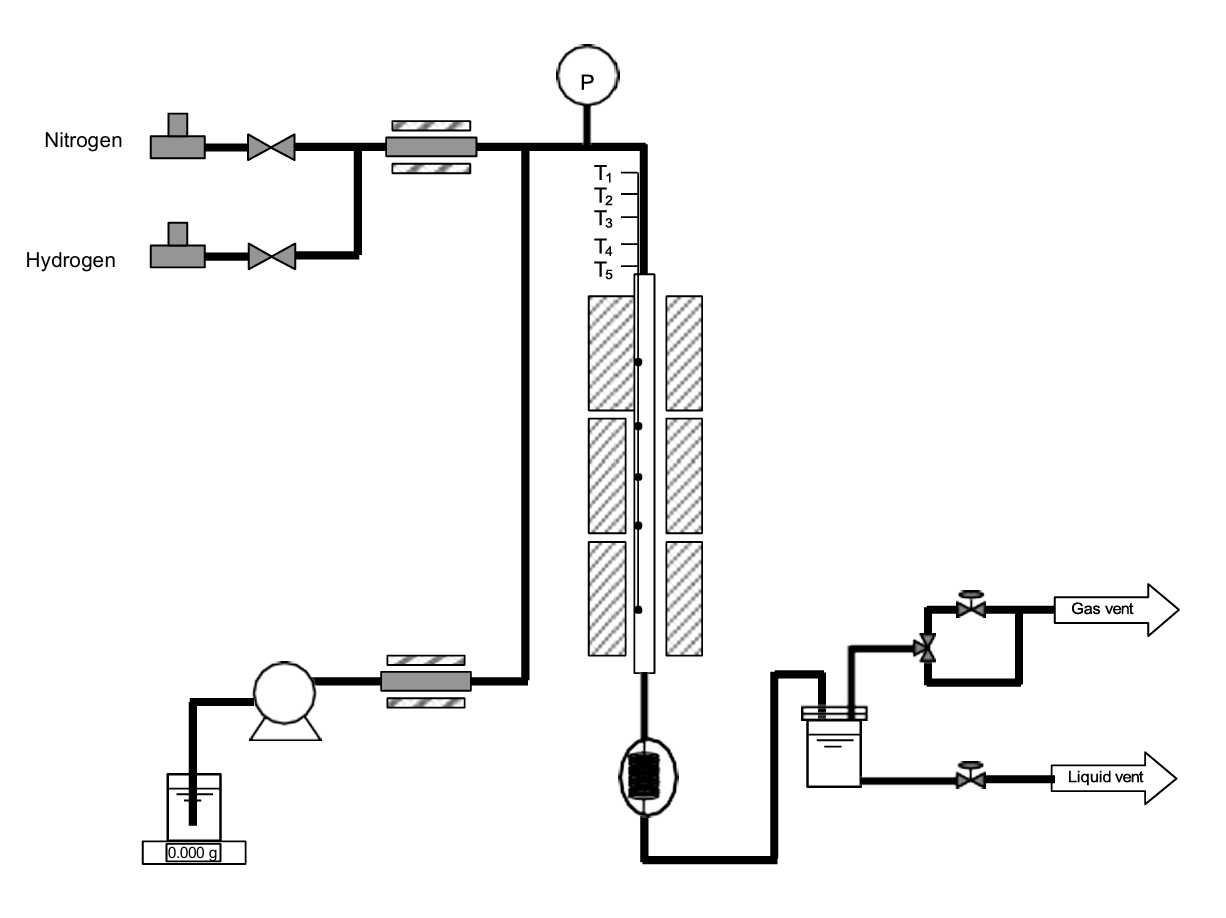
Magnetic suspension balance. Gravimetric sorption in real-world conditions. Dual-sample testing, up to 400°C.
The Fischer-Tropsch (F-T) process is perhaps the oldest and most well-known method for producing synthetic fuels1. The original process was developed in the 1920s and 1930s and was commercialized in Germany by the late 1930s.The F-T process was to produce fuel for both automobiles and military equipment.
The F-T process can be utilized to generate biofuels from nearly any carbon-containing biomass, including municipal waste, wood chips, celluloid grasses, and more. The first step in such a process is the gasification of the biomass to form Syngas (H2+CO). This Syngas is then converted into hydrocarbons through the F-T process using a catalyst, typically iron or cobalt. By carefully controlling key process parameters -such as temperature, pressure, ratio of H2 to CO-the product composition can be controlled. The F-T process can yield a wide range of hydrocarbons, from light gases to heavy waxes.
Biomass -> Gasification -> Syngas -> F-T -> Fuel
Figure 1 illustrates a typical F-T BenchCAT reactor designed by AMI. The four gases include hydrogen and carbon monoxide (Syngas), nitrogen as a diluent, and argon as an internal standard for analysis. The reactor is designed to operate at temperatures up to 400°C and pressures up to 1,500 psig, although typical operating conditions are lower. The system includes three separators to facilitate product collection:
1. The first separator, maintained at approximately 150°C, collects heavier products, such as waxes.
2. The second separator, set at 80°C, captures mid-range hydrocarbons and some water.
3. The third separator, kept at room temperature, collects lower-end hydrocarbons along with a significant amount of water.
All separation processes occur at the reactor’s operating pressure, ensuring efficient product recovery.
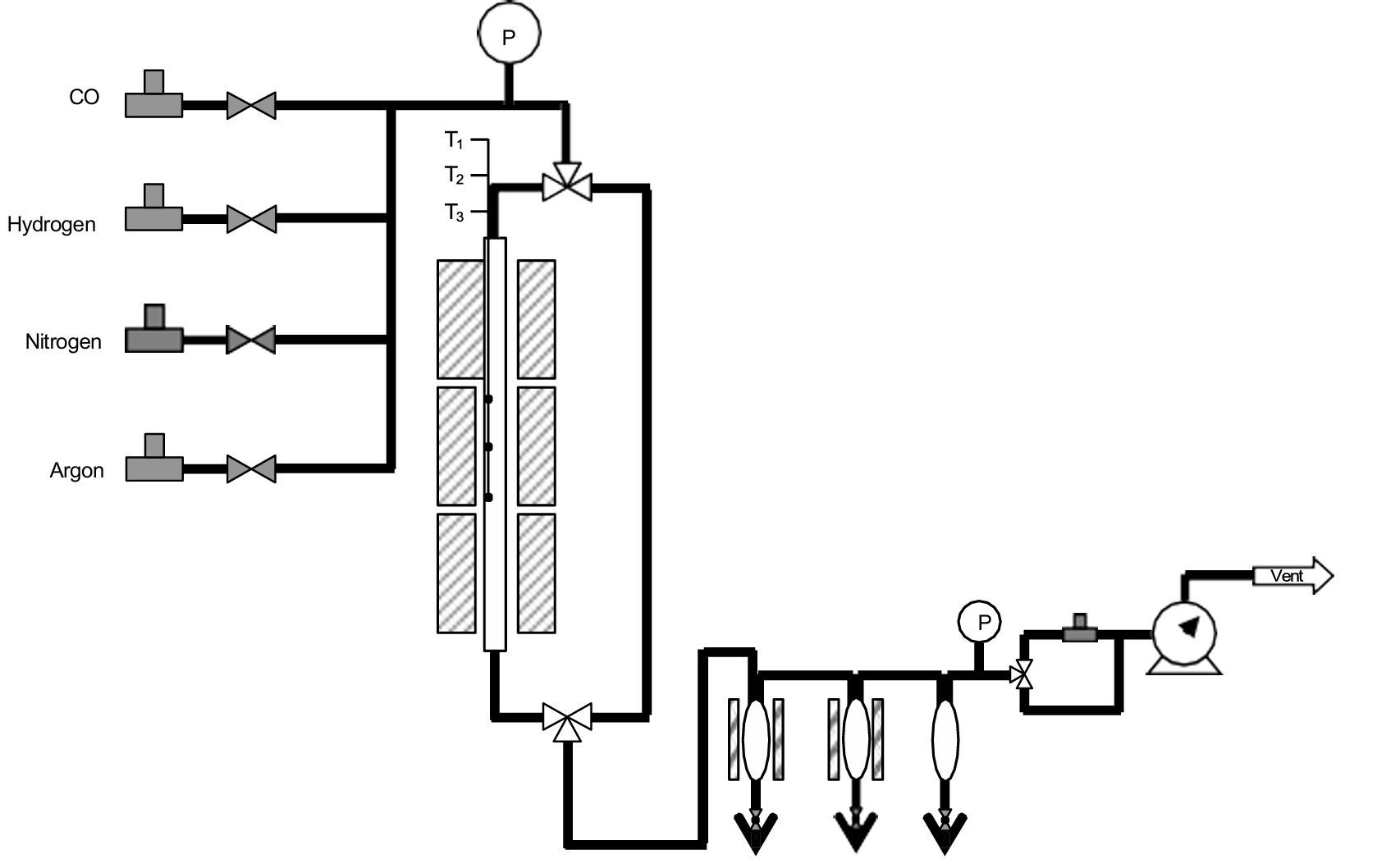
Figure1 Schematic of typical F-T BenchCAT reactor.